AI in drug development - toward fully autonomous drug discovery

In our previous blog, we noted how the increasing utilization of AI across different phases of the drug discovery process has proven its strategic value in addressing some of the core efficiency and productivity challenges involved.
As a result, AI in drug discovery and development has finally cut through the hype and become an industry-wide reality. A key milestone in this process has been the launch of clinical trials for the first drug developed completely using AI.
Currently, the rapid evolution of AI-powered protein folding algorithms, such as AlphaFold, RoseTTAFold, and RaptorX6, promises to dramatically accelerate structural biology, protein engineering, and drug discovery.
In fact, AI is expected to underpin a Million-X Drug Discovery future, wherein the ability of these technologies to exponentially scale up protein structure prediction and chemical compound generation will increase the opportunity for drug discovery by a million times.
AI-driven drug development also facilitates several other strategic outcomes such as access to larger datasets, reduced drug discovery costs, optimized drug designs, accelerated drug repurposing or repositioning, enabling the discovery of new and hidden drug targets, and turning previously undruggable targets into druggable ones.
AI Applications in Drug Design
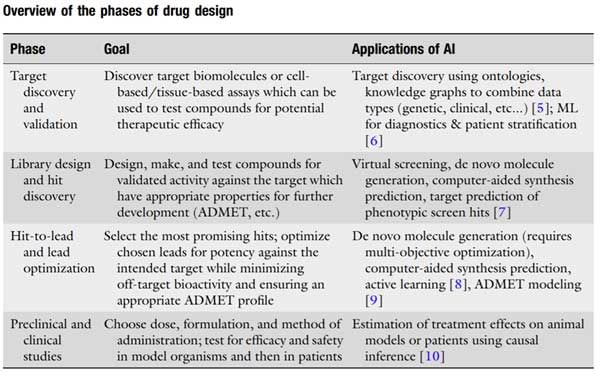
SOURCE: Springer
There are a range of applications for AI across different phases of drug development, from target discovery to clinical studies. Here’s a quick overview of how AI can transform some of the key stages of drug design:
AI in Virtual Screening
Drug discovery typically begins with the identification of targets for a disease of interest, followed by high-throughput screening (HTS) of large chemical libraries to identify bioactive compounds. Though HTS has its advantages, it may not always be appropriate or even adequate, especially in the big data era when chemical libraries have expanded beyond a billion molecules.
This is where AI-powered Virtual Screening (VS) methods are being used to complement HTS to accelerate the exploratory research process in the discovery of potential drug components. This is due to AI-based VS’s ability to rapidly screen millions of compounds at a fraction of the costs associated with HTS and with a prediction accuracy as high as 85%.
AI in Lead Optimization
Lead optimization (LO) is an essential yet expensive and time-consuming phase in preclinical drug discovery. The fundamental utility of the LO process is to enhance the desirable properties of a compound while eliminating structural deficiencies and the potential for adverse side effects. However, this is a complex multiparameter optimization problem where several competing objectives have to be precisely balanced in order to arrive at optimal drug candidates.
Done right, LO can significantly reduce the chances of attrition in pre-clinical as well as clinical stages of drug development. And reducing the iterations required for optimization in the design-make-test-analyze (DMTA) cycle can help accelerate the drug development process.
Deep learning generative models are now being successfully used to accelerate the obtention of lead compounds while simultaneously ensuring that these compounds also conform to the requisite biological objectives. Generative modeling platforms, with integrated predictive models for calculating various absorption, distribution, metabolism, excretion, and toxicity (ADMET) endpoints, can now significantly shorten the DMTA cycle required to select and design compounds that satisfy all defined LO criteria.
AI in Computer-Aided Drug Synthesis
The integration of AI and drug synthesis has been accelerated over the last few years, significantly improving the design and synthesis of drug molecules. AI-driven computer-aided synthesis tools are being widely used in retrosynthetic analysis, reaction prediction, and automated synthesis. For instance, these tools can be applied to the retrosynthetic analysis of target compounds to identify feasible synthetic routes, predict reaction products and yields, and optimize hit compounds. .
AI in computer-aided synthesis planning (CASP) is enabling chemists to objectively identify the most efficient and cost-effective synthetic route for a target molecule, thereby accelerating the ‘make’ phase of the DMTA cycle. The emergence of intelligent and automated technologies for continuous-flow chemical synthesis promises a future of fully autonomous synthesis.
These are just a few examples of the potential for AI in drug discovery and development. In fact, companies are using AI to address key challenges across the R&D pipeline and the life sciences value chain.
The Future of AI In Drug Development
According to a research paper, the future of drug discovery will entail a centralized closed-loop ML-controlled workflow that autonomously generates hypotheses, synthesizes lead candidates, tests them, and stores the data.
According to the paper, the human interface between conventional discovery processes, such as data analysis, computational prediction, and experimentation, results in bottlenecks and biased hypothesis generation, which could be eliminated by a completely automated closed-loop system.
Fully autonomous drug discovery may well be the future but in the near term, the human component will remain essential in the drug discovery and development process. In the current humans-in-the-loop approach to AI in drug design, AI algorithms are augmenting human intelligence by independently extracting and learning from patterns in vast volumes of complex big data. AI technologies like natural language processing (NLP) are helping to obtain from unstructured data sources like scientific literature, clinical trials, electronic health records (EHRs), and social media posts that have thus far remained completely underutilized.
Most importantly though, AI in drug discovery has grown far beyond hype and hypothesis. As we mentioned in our AI in drug development - from hype to reality blog, today the AI-driven drug discovery space is rife with activity as big Pharma, big tech, and big VC-funded scrappy startups jostle for a position in the next big innovation cycle in drug discovery and development.
AI-driven innovation is already delivering measurable value across the biopharma research value chain. And companies continue to scale AI across their R&D systems, bringing the industry closer to a potential future of fully autonomous drug discovery.
Subscribe to our blog:






